












Mechanical energy
It manifest in many forms,but can be broadly classified into elastic potential energy and kinetic energy.
| Mechanical energy is converted | |
|---|---|
| into | by |
| Mechanical energy | Lever |
| Thermal energy | Brakes |
| Electric energy | Dynamo |
| Electromagnetic radiation | Synchrotron |
| Chemical energy | Matches |
| Nuclear energy | Particle accelerator |
- Potential energy refers to the energy any object gets due to its position in a force field.The name "potential" energy originally signified the idea that the energy could readily be transferred as work—at least in an idealized system (reversible process, see below).
Potential energy, symbols Ep, V or Φ, is defined as the work done against a given force (= work of given force with minus sign) in changing the position of an object with respect to a reference position (often taken to be infinite separation). If F is the force and s is the displacement,
with the dot representing the scalar product of the two vectors.
- Elastic potential energyis defined as a work needed to compress (or expand) a spring. The force,F, in a spring or any other system which obeys Hooke's law is proportional to the extension or compression, x,
-
- F = − kx
where k is the force constant of the particular spring (or system). In this case, the calculated work becomes
only when k is constant. Hooke's law is a good approximation for behaviour of chemical bonds under normal conditions, i.e. when they are not being broken or formed.
- Kinetic energy, symbols Ek, T or K, is the work required to accelerate an object to a given speed.
As a ball falls freely under the influence of gravity, it accelerates downward, its initial potential energy converting into kinetic energy. On impact with a hard surface the ball deforms, converting the kinetic energy into elastic potential energy. As the ball springs back, the energy converts back firstly to kinetic energy and then as the ball re-gains height into potential energy. Energy conversion to heat due to inelastic deformation and air resistance cause each successive bounce to be lower than the last.
Surface energy
A minimal surface, for example, represents the smallest possible energy that a surface can have if its energy is proportional to the area of the surface. For this reason, (open) soap films of small size are minimal surfaces (small size reduces gravity effects, and openness prevents pressure from building up. Note that a bubble is a minimum energy surface but not a minimal surface by definition).
Sound energy
Sound is a form of mechanical vibration which propagates through any mechanical medium.
Gravitational energy
The gravitational force near the Earth's surface varies very little with the height, h, and is equal to the mass, m, multiplied by the gravitational acceleration, g = 9.81 m/s². In these cases, the gravitational potential energy is given by
-
- Ep,g = mgh
A more general expression for the potential energy due to Newtonian gravitation between two bodies of masses m1 and m2, useful in astronomy, is
-
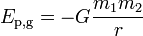 ,
,
where r is the separation between the two bodies and G is the gravitational constant, 6.6742(10)×10−11 m3kg−1s−2. In this case, the reference point is the infinite separation of the two bodies.
Thermal energy
It is the energy associated with the microscopical random motion of particles constituting the media.
A heat is defined as a transfer (flow) of thermal energy across certain boundary (for example, from a hot body to cold via the area of their contact. A practical definition for small transfers of heat is
where Cv is the heat capacity of the system. This definition will fail if the system undergoes a phase transition—e.g. if ice is melting to water—as in these cases the system can absorb heat without increasing its temperature. In more complex systems, it is preferable to use the concept of internal energy rather than that of thermal energy.
Electric energy
Electrostatic energy
The electric potential energy of given configuration of charges is defined as the work which must be done against the Coulomb force to rearrange charges from infinite separation to this configuration (or the work done by the Coulomb force separating the charges from this configuration to infinity).
Electric energy
If an electric current passes through a resistor, electric energy is converted to heat; if the current passes through an electric appliance, some of the electric energy will be converted into other forms of energy (although some will always be lost as heat).
Magnetic energy
There is no fundamental difference between magnetic energy and electric energy: the two phenomena are related by Maxwell's equations. The potential energy of a magnet of magnetic moment m in a magnetic field B is defined as the work of magnetic force (actually of magnetictorque) on re-alignment of the vector of the magnetic dipole moment.
Chemical energy
It is the energy due to associations of atoms in molecules and various other kinds of aggregates of matter. If the chemical energy of a system decreases during a chemical reaction, the difference is transferred to the surroundings in some form (often heat or light); on the other hand if the chemical energy of a system increases as a result of a chemical reaction - the difference then is supplied by the surroundings (usually again in form of heat or light).
Nuclear energy
Nuclear potential energy, along with electric potential energy, provides the energy released from nuclear fission and nuclear fusion processes. The result of both these processes are nuclei in which the more-optimal size of the nucleus allows the nuclear force (which is opposed by the electromagnetic force) to bind nuclear particles more tightly together than before the reaction.
The Weak nuclear force (different from the strong force) provides the potential energy for certain kinds of radioactive decay, such as beta decay.
The energy released in nuclear processes is so large that the relativistic change in mass (after the energy has been removed) can be as much as several parts per thousand.
Nuclear particles (nucleons) like protons and neutrons are not destroyed (law of conservation of baryon number) in fission and fusion processes. A few lighter particles may be created or destroyed (example: beta minus and beta plus decay, or electron capture decay), but these minor processes are not important to the immediate energy release in fission and fusion. Rather, fission and fusion release energy when collections of baryons become more tightly bound, and it is the energy associated with a fraction of the mass of the nucleons (but not the whole particles) which appears as the heat and electromagnetic radiation generated by nuclear reactions. This heat and radiation retains the "missing" mass, but the mass is missing only because it escapes in the form of heat or light, which retain the mass and conduct it out of the system where it is not measured.












0 comments:
Post a Comment