Sugar you eat is "combusted" by your body to produce CO2 and H2O. During this process energy is also released.
This energy is used (among other things) to:
- Operate your muscles
- Maintain your body temperature
Chemical reactions involve changes in energy:
- Some reactions produce energy
- Some reactions require energy
Our society as an "organism" requires energy: 90% of our energy comes from chemical reactions involving the combustion of petroleum products.
The study of energy and its transformations is known as thermodynamics
The relationship between chemical reactions and energy changes is known as thermochemistry.
The Nature of Energy
A Force is any kind of push or pull exerted on an object.
- Gravity is a force which keeps us stuck to the earth.
- The Electrostatic force attracts electrons to protons in an atom.
If you move an object against some force, work is being done.
The amount of work (w) being done is relative to the distance (d) the object is moved and the strength of the force (F) against the object:
w = F * d
Energy, in the form of work, must be used to move an object against a force.
When we do work, our body temperature increases (and we sweat to cool us down). Our bodies are generating Heat energy.
Heat is an energy which is transferred from one object to another depending on the relative temperature:
- Heat energy flows from an object towards other objects of lower temperature
Energy is the capacity to do work or to transfer heat
Objects can possess energy due to their motions and positions, as kinetic energy and potential energy.
Kinetic and Potential Energy
Kinetic energy is the energy of motion. The magnitude of the kinetic energy (Ek) of an object depends upon its mass (m) and velocity (v):
Potential energy is the result of the attractions and repulsion between objects. An electron has potential energy when located near a proton due to the attractive electrostatic force between them.
- Chemical energy is the potential energy stored in the arrangement of electrons and protons.
- Thermal energy reflects the kinetic energy of the molecules of a substance.
Energy Units
The SI unit for energy is the joule ("J"). In honor of James Prescot Joule (1818-1889) a British Scientist who investigated work and heat. (Note: SI is short for the French term Systeme International d'Unites. Which defines metric standards).
Kinetic energy for example is defined as:
Thus, the joule must have units of:
kg*(meters/second)2
and, in fact, 1 joule is defined as:
Traditionally, energy changes accompanying chemical reactions have been expressed in calories, which is a non-SI unit (though still widely used).
1 calorie = 4.184 J


























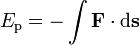


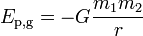 ,
,

